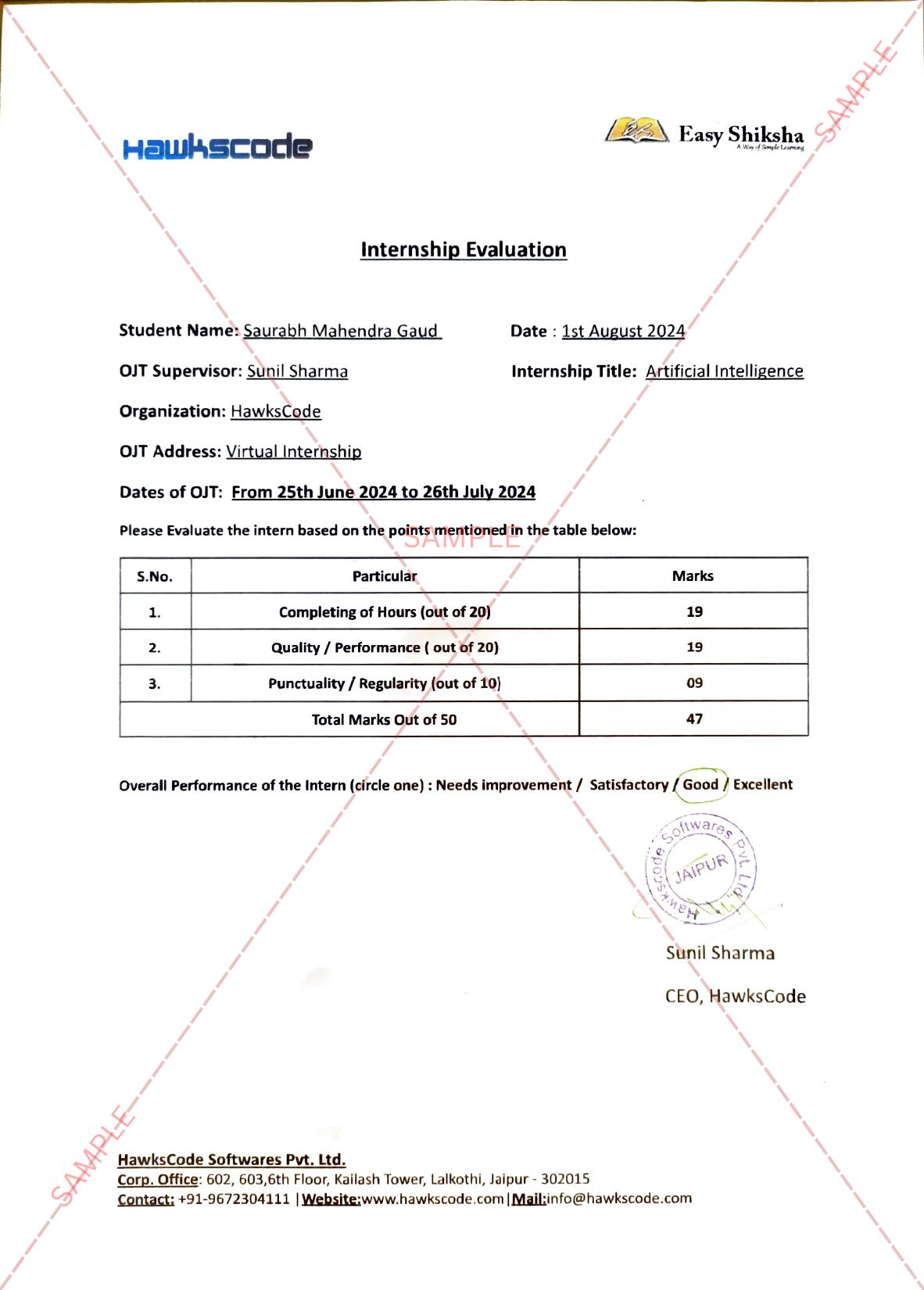
- Glenmark is the first company in India to have received the regulator’s approval for 400 mg dosage form
- Increased strength of FabiFlu® yet another milestone effort by Glenmark’s in-house R&D
- Patients can now opt for a more relaxed dosage regimen when compared to 200 mg tablet and now need to take half the number of pills due to the introduction of 400 mg
- Glenmark remains the only company in India to successfully complete an randomized, controlled, open-labelled, multi-center Phase 3 clinical trial on Indian patients with mild to moderate Covid-19
India; August, 2020:Glenmark Pharmaceuticals, a research-led, integrated global pharmaceutical company, today announced that it will introduce a 400 mg version of oral antiviral FabiFlu®, for the treatment of mild to moderate COVID-19 in India. The higher strength will improve patient compliance and experience, by effectively reducing the number of tablets that patients require per day.

Important Announcement – EasyShiksha has now started Online Internship Program “Ab India Sikhega Ghar Se”
Important Announcement – EasyShiksha has now started Online Internship Program “Ab India Sikhega Ghar Se” during this lockdown.
A higher pill burden has been associated with lower adherence to therapy, the latter affecting viral suppression and overall treatment outcomes. Also reducing the pill burden has been a demand from doctors and patients to enable adherence. The 200 mg dosage of FabiFlu® required patients to take 18 tablets on Day 1 (nine in the morning and nine in the evening), followed by 8 tablets each day thereafter for a maximum of 14 days. With the new 400 mg version, patients will now have a more relaxed dosage regimen, with 9 tablets required on Day 1( 4.5 in the morning and 4.5 in the evening), and thereafter 2 tablets twice a day from Day 2 till end of the course.
Top Courses in Software Engineering
Explaining the significance of this development, Dr. Monika Tandon, Vice President & Head, Clinical Development, Global Specialty/Branded Portfolio, Glenmark Pharmaceuticals Ltd., said, “Being the first company to launch Favipiravir in India, we continue to innovate and seek new treatment options for Covid-19 patients. Introducing this higher strength of FabiFlu® is in line with these efforts to ensure a smoother experience for patients, by reducing their daily pill burden.”
“The 200 mg dosage of FabiFlu® was developed in line with global formulations of the drug Favipiravir, which had similar strength. The 400 mg version is a result of Glenmark’s own R&D efforts to improve treatment experience for patients in India,” she added.
Empower your team. Lead the industry
Get a subscription to a library of online courses and digital learning tools for your organization with EasyShiksha
Request NowQ. Are EasyShiksha's internships truly free?
Yes, all internships offered by EasyShiksha are completely free of charge.
Q. How can I apply for an internship with EasyShiksha?
You can apply by visiting our website, browsing available internships, and following the application instructions provided.
Q. What types of internships are available through EasyShiksha?
EasyShiksha offers a wide range of internships across technology, business, marketing, healthcare, and more. Opportunities are continuously updated.
Q. Will I receive a certificate upon completing an internship?
Yes, upon successful completion, you will receive a certificate recognizing your participation and achievements.
Q. Are EasyShiksha's internship certificates recognized by universities and employers?
Yes, the certificates are recognized by universities, colleges, and employers worldwide.
Q. Is the download of certificates free or paid?
Access to internships and courses is free, but there is a small fee to download certificates, covering administrative costs.
Q. When can I start the course?
You can choose any course and start immediately without delay.
Q. What are the course and session timings?
These are fully online courses. You can learn at any time and pace. We recommend following a routine, but it depends on your schedule.
Q. What will happen when my course is over?
After completion, you will have lifetime access to the course for future reference.
Q. Can I download the notes and study material?
Yes, you can access and download course materials and have lifetime access for future reference.
Q. What software/tools would be needed for the course?
All necessary software/tools will be shared during the training as needed.
Q. I’m unable to make a payment. What should I do?
Try using a different card or account. If the problem persists, email us at info@easyshiksha.com.
Q. Do I get the certificate in hard copy?
No, only a soft copy is provided, which can be downloaded and printed if required.
Q. The payment got deducted but shows “failed”. What to do?
Technical errors may cause this. The deducted amount will be returned to your account in 7-10 working days.
Q. Payment was successful but dashboard shows ‘Buy Now’?
Sometimes payment reflection is delayed. If it takes longer than 30 minutes, email info@easyshiksha.com with the payment screenshot.
Q. What is the refund policy?
If you face technical issues, you can request a refund. No refunds are issued once the certificate has been generated.
Q. Can I enroll in a single course?
Yes, select the course of interest, fill in the details, make payment, and start learning. You will also earn a certificate.
Q. My questions are not listed above. I need further help.
Contact us at info@easyshiksha.com for further assistance.
Glenmark has also commenced a Post Marketing Surveillance (PMS) study on FabiFlu® to closely monitor the efficacy and safety of the drug in a large pool of patients prescribed with the oral antiviralFavipiravir, as part of an open label, multicenter, single arm study.Glenmark is also conducting another Phase 3 clinical trial to evaluate the efficacy of two antivirals drugs Favipiravir and Umifenovir as a combination therapy in moderate hospitalized adult COVID-19 patients in India. The combination study which is called the FAITH trial is looking to enrol 158 hospitalized patients of moderate COVID-19 in India. Early treatment with combination therapy will be evaluated for safety and efficacy as it is emerging as an effective approach in shortening duration of virus shedding, facilitating early clinical cure and discharge of patients.
For more information related to technology, visit HawksCode and EasyShiksha
ALSO READ: puneet-vashists-path-breaking-entry-lord-shani-tvs-kahat
Get Course: gain-control-over-your-finances-and-save-capital