- Emergency Use Listing by WHO will expedite global access and availability of COVAXIN® worldwide.
- The WHO’s Emergency Use Listing (EUL) for COVAXIN® facilitates countries to expedite their regulatory approval processes to introduce and administer India’s indigenously made COVID-19 vaccine developed and manufactured by Bharat Biotech.
- It also allows procurement by UNICEF, PAHO, and the GAVI COVAX facility for distribution to countries in need.
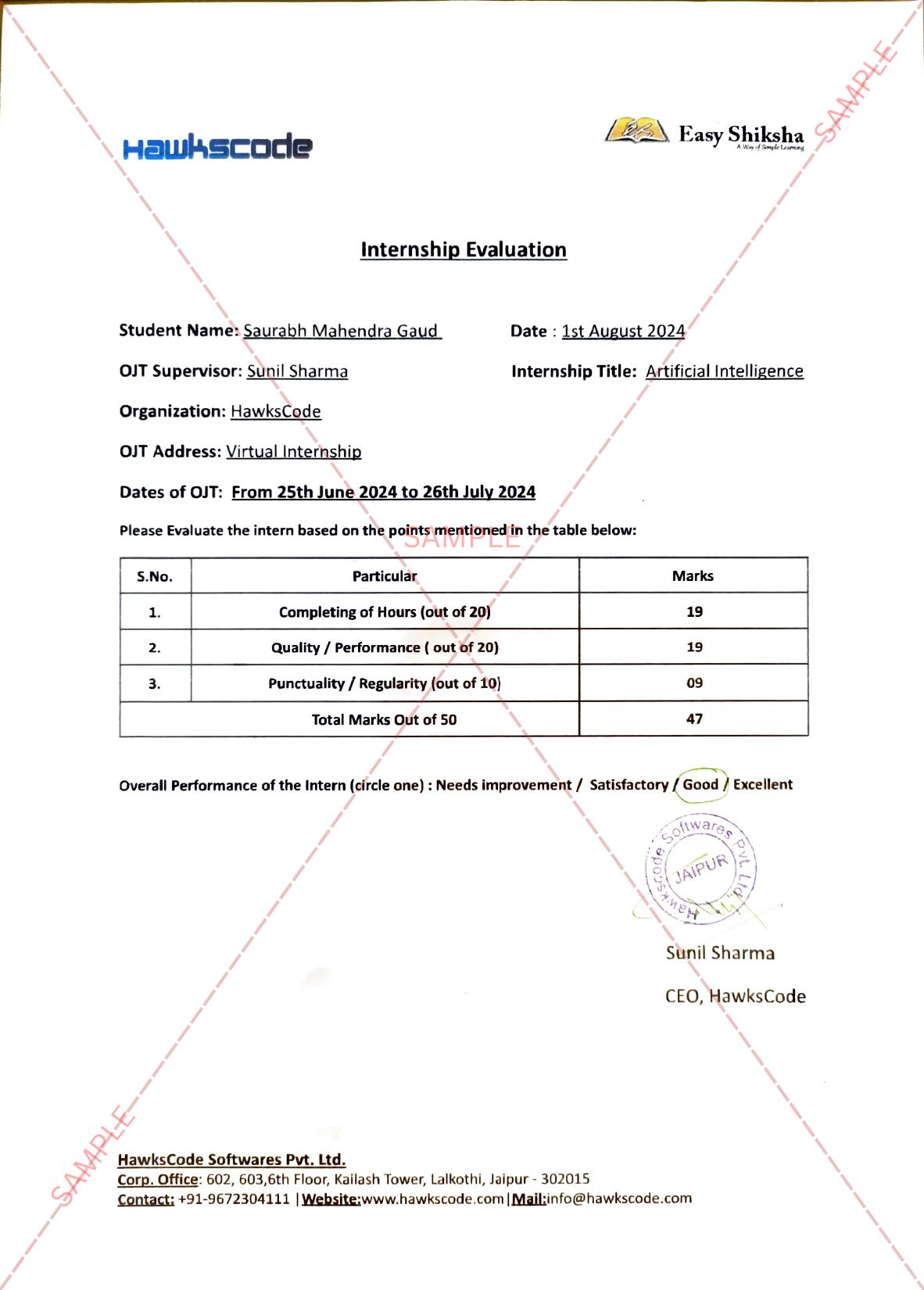
Hyderabad, November 03, 2021: Bharat Biotech, a global leader in vaccine development and innovation, today announced that COVAXIN®, India’s first indigenous Covid-19 vaccine, has been granted Emergency Use Listing by the World Health Organization (WHO). This vaccine is a whole virion-inactivated vaccine against SARS-CoV2, developed in partnership with ICMR and NIV, Pune. The emergency use listing (EUL) procedure assesses the suitability of novel health products during public health emergencies, with the objective of providing rapid access to medicines, vaccines, and diagnostics while adhering to stringent criteria of safety, efficacy, and quality.

Important Announcement – EasyShiksha has now started Online Internship Program “Ab India Sikhega Ghar Se”
With validation from the WHO, countries can now expedite their regulatory approval processes to import and administer COVAXIN®. Furthermore, UNICEF, Pan-American Health Organization (PAHO), GAVI COVAX facility, will be able to procure COVAXIN® for distribution to countries worldwide. This will enable them to secure the critical supply needed to meet the requirements of priority populations, thereby ensuring equitable access.
Dr. Krishna Ella, Chairman and Managing Director, Bharat Biotech, said, “Validation by WHO is a very significant step towards ensuring global access to India’s widely administered, safe, and efficacious COVAXIN®. As an organization, we have focused on maintaining stringent quality and safety standards that meet rigorous assessment, and scientific standards established by WHO, as a result, many of our vaccines have received WHO prequalification. The EUL authorization for COVAXIN® will enable us to contribute to accelerating the equitable access of Covid-19 vaccine, and the access to our vaccine globally thereby addressing the current public health emergency.”
The phase 3 trial data for COVAXIN® was available during June 2021. The World Health Organisation (WHO) Emergency Use Listing (EUL) process commenced on July 6, 2021, with rolling data submission. The Strategic Advisory Group of Experts on Immunization (SAGE) of WHO had reviewed COVAXIN® data on October 5 in a meeting and granted EUL for COVAXIN® today (November 3, 2021). COVAXIN® has been specifically designed to meet the needs of global distribution chains, the requirements for which are more critical in low- and middle-income countries. It has been formulated to enable shipping and long-term storage at 2-8ºC. It is also formulated to adhere to a multi-dose vial policy, thereby reducing open vial wastage, saving money to procurement agencies and governments alike.
Mrs. Suchitra Ella, Joint Managing Director, Bharat Biotech, said, “The WHO nod for COVAXIN® is a validation of the tremendous effort made by everyone at Bharat Biotech and our partners. It is also an opportunity for us to create a meaningful impact at a global level. Such an impact can only be orchestrated when multiple stakeholders come together to work towards a common goal. COVAXIN® is a great example of a successful public-private partnership in developing a world-class COVID-19 vaccine. We look forward to playing a larger role to help the efforts by both the developed and developing nations to control the pandemic.”
COVAXIN® has been evaluated through neutralizing antibody responses against several variants of concern, namely B.1.617.2 (Delta), B.1.617.1 (Kappa), B.1.1.7 (Alpha), B.1.351 (Beta), P2- B.1.1.28 (Gamma). The data from these studies and others have been extensively published in more than 12 peer-reviewed journals and are available for review in the public domain.
Top Courses in Software Engineering
Manufacturing capacity expansion was started during Q1 2021, as the first phase, 3 efficacy readouts were available. Within a short period of ~9 months, the capacity was scaled up to 50-55 million doses per month, as of October 2021. Bharat Biotech has established COVAXIN® manufacturing to reach an annualized capacity of 1 billion doses by the end of 2021. Technology transfer activities are also in progress to companies in India, the United States, and other countries.
More about COVAXIN ® – https://www.bharatbiotech.com/covaxin.html

About Bharat Biotech
Bharat Biotech has established an excellent track record of innovation with more than 145 global patents, a wide product portfolio of more than 16 vaccines, 4 bio-therapeutics, registrations in more than 123 countries, and the World Health Organization (WHO) Pre-qualifications. Located in Genome Valley in Hyderabad, India, a hub for the global biotech industry, Bharat Biotech has built a world-class vaccine & bio-therapeutics, research & product development, Bio-Safety Level 3 manufacturing, and vaccine supply and distribution. Having delivered more than 4 billion doses of vaccines worldwide, Bharat
Biotech continues to lead innovation and has developed vaccines for influenza H1N1, Rotavirus, Japanese Encephalitis (JENVAC®), Rabies, Chikungunya, Zika, Cholera, and the world’s first tetanus-toxoid conjugated vaccine for Typhoid. Bharat’s commitment to global social innovation programs and the public-private partnership resulted in introducing path-breaking WHO pre-qualified vaccines BIOPOLIO®, ROTAVAC®, and Typbar TCV® combatting polio, rotavirus, typhoid infections, respectively.
The acquisition of Chiron Behring Vaccines has positioned Bharat Biotech as the world’s largest rabies vaccine manufacturer with Chirorab® and Indirab®.
To learn more about Bharat Biotech, visit www.bharatbiotech.com.
For more information, please contact:
Sheela Panicker enright@enrightpr.com / +91 984-980-9594
Sanjay Chaudhary Sanjay.choudhry@perfectrelations.com / +91 852-700-0614
Shilpa Suryawanshi shilpa.suryawanshi@perfectrelations.com / +91 983-373-8595
For more related content visit Easyshiksha and Hawkscode
Empower your team. Lead the industry
Get a subscription to a library of online courses and digital learning tools for your organization with EasyShiksha
Request NowQ. Are EasyShiksha's internships truly free?
Yes, all internships offered by EasyShiksha are completely free of charge.
Q. How can I apply for an internship with EasyShiksha?
You can apply by visiting our website, browsing available internships, and following the application instructions provided.
Q. What types of internships are available through EasyShiksha?
EasyShiksha offers a wide range of internships across technology, business, marketing, healthcare, and more. Opportunities are continuously updated.
Q. Will I receive a certificate upon completing an internship?
Yes, upon successful completion, you will receive a certificate recognizing your participation and achievements.
Q. Are EasyShiksha's internship certificates recognized by universities and employers?
Yes, the certificates are recognized by universities, colleges, and employers worldwide.
Q. Is the download of certificates free or paid?
Access to internships and courses is free, but there is a small fee to download certificates, covering administrative costs.
Q. When can I start the course?
You can choose any course and start immediately without delay.
Q. What are the course and session timings?
These are fully online courses. You can learn at any time and pace. We recommend following a routine, but it depends on your schedule.
Q. What will happen when my course is over?
After completion, you will have lifetime access to the course for future reference.
Q. Can I download the notes and study material?
Yes, you can access and download course materials and have lifetime access for future reference.
Q. What software/tools would be needed for the course?
All necessary software/tools will be shared during the training as needed.
Q. I’m unable to make a payment. What should I do?
Try using a different card or account. If the problem persists, email us at info@easyshiksha.com.
Q. Do I get the certificate in hard copy?
No, only a soft copy is provided, which can be downloaded and printed if required.
Q. The payment got deducted but shows “failed”. What to do?
Technical errors may cause this. The deducted amount will be returned to your account in 7-10 working days.
Q. Payment was successful but dashboard shows ‘Buy Now’?
Sometimes payment reflection is delayed. If it takes longer than 30 minutes, email info@easyshiksha.com with the payment screenshot.
Q. What is the refund policy?
If you face technical issues, you can request a refund. No refunds are issued once the certificate has been generated.
Q. Can I enroll in a single course?
Yes, select the course of interest, fill in the details, make payment, and start learning. You will also earn a certificate.
Q. My questions are not listed above. I need further help.
Contact us at info@easyshiksha.com for further assistance.
ALSO READ: Directorate-of-public-relations-government-of-madhya-pradesh
Get Course: Project-Management-for-Beginners